Lab 3: Solubility of Organic Compounds
Objectives:
Introduction:
The solubility of a solute (a dissolved substance) in a solvent (the dissolving medium) is the most important chemical principle underlying three major techniques you will study in the organic chemistry laboratory: crystallization, extraction, and chromatography. In this experiment on solubility you will gain an understanding of the structural features of a substance that determine its solubility in various solvents. This understanding will help you to predict solubility behavior and to understand the techniques that are based on this property.
In one part of this experiment, you will determine whether a solid organic compound is
soluble
or
insoluble
in a given solvent. You should keep in mind that this is actually an over simplification since some solids may be
partially soluble
in a given solvent. If the organic compound being dissolved in a solvent is a liquid, then it is sometimes more appropriate to say that the compound and the solvent are
miscible
(mix homogeneously in all proportions). Likewise, if the liquid organic compound is insoluble in the solvent, then they are
immiscible
(do not mix, and form two liquid phases).
A major goal of this experiment is to learn how to make predictions about whether or not a substance will be soluble in a given solvent. This is not always easy to do, even for an experienced chemist. However there are some guidelines which will often make it possible for you to make a good guess about the solubilities of compounds in specific solvents. In discussing these guidelines it is helpful to separate the types of solutions we will be looking at into two categories: (1) Solutions in which both the solvent and the solute are covalent (molecular). (2) Ionic solutions in which the solute ionizes and dissociates.
I. Solutions in which the Solvent and Solute are Molecular
A generalization which is very useful in predicting solubility behavior is the widely used rule,
"Like dissolves like."
This rule is most commonly applied to polar and nonpolar compounds. According to this rule, a polar solvent will dissolve polar (or ionic) compounds and a nonpolar solvent will dissolve nonpolar compounds.
The reason why this rule works has to do with the nature of intermolecular forces of attraction. Although we will not be focusing on the nature of these forces, it is helpful to know what they are called. The force of attraction between polar molecules is called dipole-dipole interaction (or hydrogen bonding under certain circumstances); between nonpolar molecules it is called London dispersion forces. In both cases these attractive forces can occur between molecules of the same compound or different compounds
In this experiment, you will be testing the solubilities of several compounds in several solvents. You will then be asked to explain these results primarily in terms of the polarity of the solvent and the solute. This can be done only if you are able to determine whether a substance is polar or nonpolar.
The polarity of a compound is dependent on both the polarities of the individual bonds and the shape of the molecule. For most organic compounds, evaluating these factors can become quite complicated because of the complexities of the molecules. However, it is possible to make some reasonable predictions by just looking at the types of atoms which a compound possesses. As you read the following guidelines, it is important to understand that, although we often describe compounds as being polar or nonpolar, polarity is a matter of degree, ranging from nonpolar to highly polar.
Guidelines.
These guidelines will be helpful in completing this laboratory experiment. They will help you to determine if a substance is polar or nonpolar and to make predictions about solubility.
1.
All hydrocarbons are nonpolar.
Examples:
petroleum ether (2-methylpentane)

Benzene
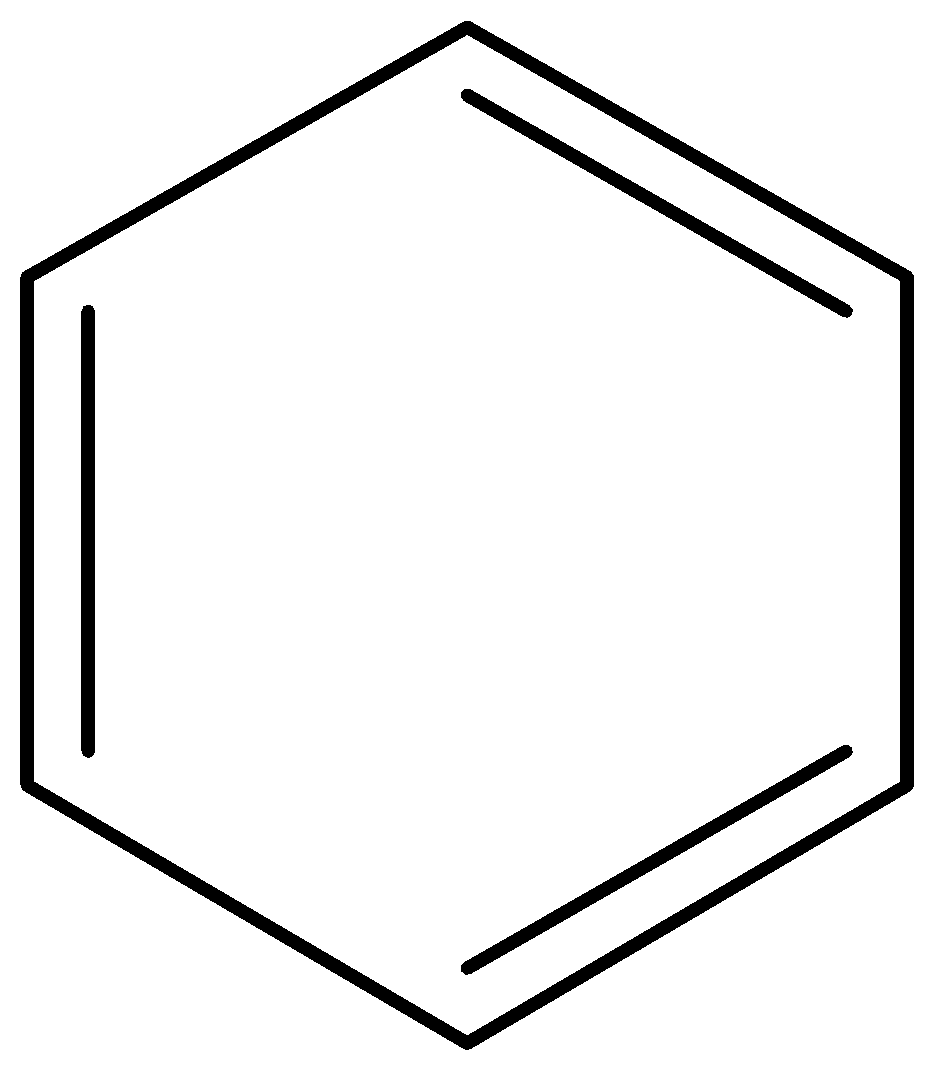
2.
Compounds possessing the electronegative elements oxygen or nitrogen are generally polar.
Examples:
Acetone

Ethyl alcohol

Ethyl acetate

Ethylamine
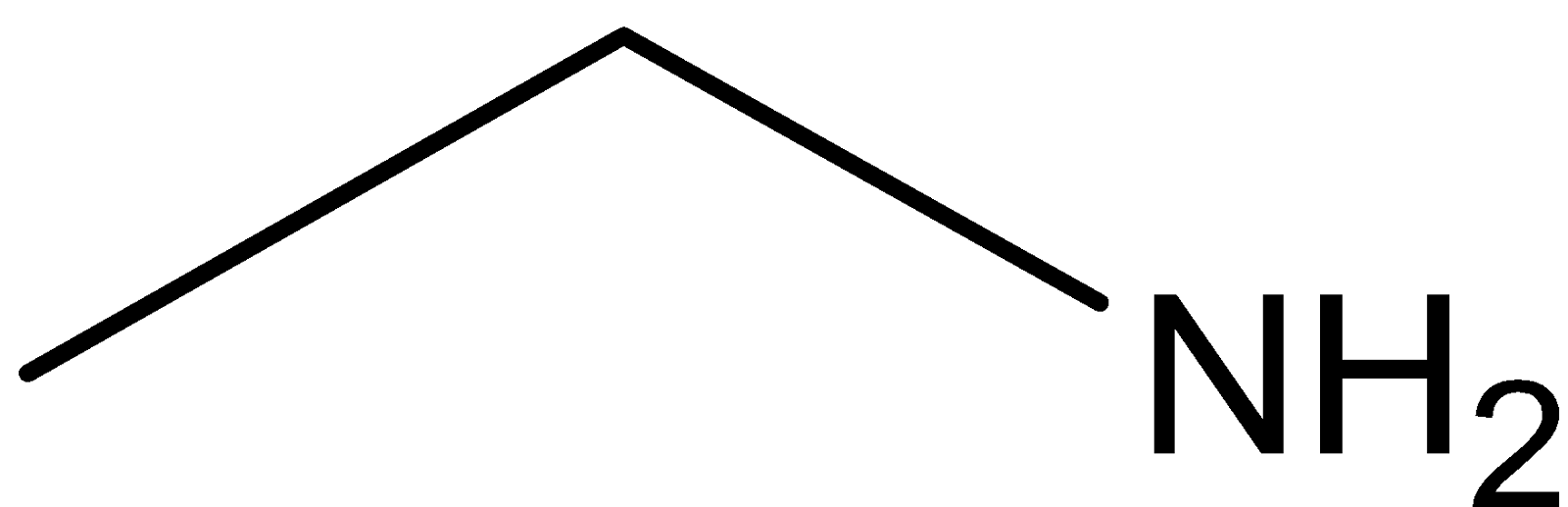
Diethyl ether

Water

The polarity of these compounds depends on the presence of polar C-O, C=O, OH, NH and CN bonds. The compounds which are most polar are capable of forming hydrogen bonds (see #4) and have NH or OH bonds. Although all of these compounds are polar, the degree of polarity ranges from slightly polar to highly polar. This is due to the effect of the size of the carbon chain on polarity and whether or not the compound can form hydrogen bonds.
3.
The presence of halogen atoms, even though their electronegativities are relatively high, does not alter the polarity of an organic compound in a significant way. Therefore, these compounds are only slightly polar. The polarities of these compounds are more similar to hydrocarbons, which are nonpolar, than to water which is highly polar.
Examples
Methylene chloride

Chlorobenzene

(dichloromethane)
4.
When comparing organic compounds within the same family, adding carbon atoms to the chain decreases the polarity. For example, methyl alcohol (CH
3
OH) is more polar than propyl alcohol (CH
3
CH
2
CH
2
OH). This is because hydrocarbons are nonpolar, and increasing the length of a carbon chain makes the compound more hydrocarbon-like. The general rule of thumb is that each polar group (groups containing nitrogen or oxygen) will allow up to 4 carbons to be soluble in water.
5.
As mentioned earlier, the force of attraction between polar molecules is dipole-dipole interaction. A special case of dipole-dipole interaction is hydrogen bonding. Hydrogen bonding is a possibility when a compound possesses a hydrogen atom bonded to a nitrogen, oxygen, or fluorine atom. It is the attraction between this hydrogen atom and a nitrogen, oxygen, or fluorine atom in another molecule. Hydrogen bonding may occur between two molecules of the same compound or between molecules of different compounds.
Hydrogen bonding is the strongest type of dipole-dipole interaction. When hydrogen bonding between solute and solvent is possible, solubility is greater than one would expect for compounds of similar polarity that cannot form hydrogen bonds. Hydrogen bonding is very important in organic chemistry and biochemistry, and you should be alert for situations in which hydrogen bonding may occur.
II. Solutions in which the Solute Ionizes and Dissociates
Many ionic compounds are highly soluble in water because of the strong attraction between ions and the highly polar water molecules. This also applies to organic compounds that can exist as ions. For example, sodium acetate consists of Na
+
and CH
3
COO
-
ions, which are highly soluble in water. Although
there are some exceptions, you may assume in this experiment that all organic compounds that are in the ionic form will be water-soluble.
The most common way by which organic compounds become ions is in acid-base reactions. For example, carboxylic acids can be converted to water-soluble salts when they react with dilute aqueous NaOH:
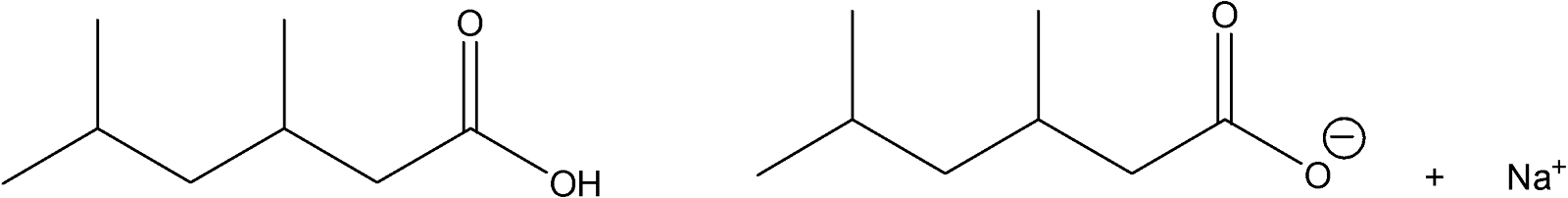
water-insoluble
carboxylic acid
water-soluble salt
The water-soluble salt can then be converted back to the original carboxylic acid by adding another acid (usually aqueous HCl) to the solution of the salt:

Amines, which are organic bases, can also be converted to water-soluble salts when they react with dilute aqueous HCl:
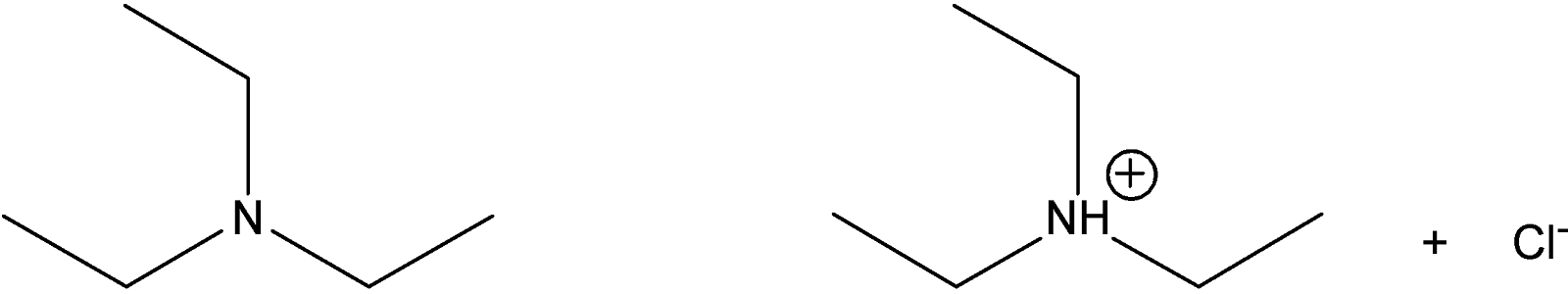
amine
water-soluble salt
This salt can be converted back to the original amine by adding a base (usually aqueous NaOH) to the solution of the salt.

REPORT – SOLUBILITY
DUE:
Name:
Due at the beginning of the next lab for credit! Pre-lab quiz due before lab in Canvas!
Lab Report Guide (20 pts total):
1. Results
2. Post Lab Questions
Part A. Solubility of Solid Compounds
Place about 40 mg (0.040 g) of biphenyl into each of two dry test tubes. (Don't try to be exact: you can be 1-2 mg off and the experiment will still work.) Label the test tubes and then add 1 mL of water to the 1st tube and 1 mL of petroleum ether to the 2nd tube. Determine the solubility of each sample in the following way. Using the rounded end of a spatula, stir each sample continuously for 60 seconds by twirling the spatula rapidly. After 60 seconds (do not stir longer), note whether the compound is soluble (dissolves completely) or insoluble (none of it dissolves). If all but a couple granules have dissolved, then you should state that the sample is soluble. Record these results in the following table. Now repeat the directions given above for with malonic acid. Record these results.
Compound
|
Water Prediction
|
Water Result
|
petroleum ether Prediction
|
petroleum ether Result
|
Biphenyl

|
in
soluble
|
insoluble
|
soluble
|
soluble
|
Malonic acid

|
soluble
|
soluble
|
soluble
|
Partially
soluble
|
Problems
1.
For each of the four solubility tests, explain your results in terms of the polarities.
a. Biphenyl in water
b. Biphenyl in petroleum ether
c. Malonic acid in water
d. Malonic acid in petroleum ether
2.
Is hydrogen bonding possible for any of the 4 pairs of solids and solvent? If so, draw a picture showing a hydrogen bond between the solvent and solute.
Part B. Solubility of Different Alcohols
For each solubility test (see table), add 1 mL of solvent (water or petroleum ether) to a test tube. Then add one of the alcohols dropwise. Shake the tube after adding each drop. Continue adding the alcohol until you have added a total of 10 drops. If you see one layer, the liquids are miscible (soluble). If you see two layers, they are immiscible. Record your results (miscible or immiscible) in your notebook in table form.
Compound
|
Water Prediction
|
Water Result
|
petroleum ether Prediction
|
petroleum ether Result
|
Methyl alcohol

|
miscible
|
miscible
|
miscible
|
immiscible
|
1-Octanol

|
immiscible
|
immiscible
|
miscible
|
miscible
|
Problems
1.
Explain each of these results.
a. methyl alcohol in water
b. methyl alcohol in petroleum ether
c. 1-octanol in water
d. 1-octanol in petroleum ether
Part C. Miscible or Immiscible Pairs
For each of the following pairs of liquid compounds, predict if the pairs will be miscible or immiscible. (It is best to make your prediction for each pair
after
you have tested the previous pairs.) Then add 1 mL of both liquids to the same test tube. Use a different test tube for each pair. Shake the test tube for 10-20 seconds to determine if the two liquids are miscible (form one layer) or immiscible (form two layers). Record your results in the table.
Mixture
|
Prediction
|
Result
|
Water and Ethyl Alcohol
|
miscible
|
miscible
|
Water and Diethyl Ether
|
immiscible
|
immiscible
|
Water and Methylene Chloride
|
|
immiscible
|
Water and petroleum ether
|
immiscible
|
immiscible
|
petroleum ether and Methylene Chloride
|
immiscible
|
miscible
|
Problems
1.
If any of your predictions were wrong, explain why.
2.
For which of the 5 pairs of liquid, is hydrogen bonding possible between the two different compounds? (More than one may be correct.)
Part D. Solubility of Organic Acids and Bases
Place about 30 mg (0.030 g) of benzoic acid into each of two dry test tubes. Label the test tubes and then add 1 mL of water to the 1st tube and 1 mL of 1.0 M NaOH to the 2nd tube. Shake each tube for 10-20 seconds. Note whether the compound is soluble (dissolves completely) or is insoluble (none of it dissolves). Record these results in table. Now take the tube containing benzoic acid and 1.0 M NaOH. Add 0.5 mL of 6.0 M HCl to the mixture and shake the tube for several seconds. Record this result.
Compound
|
Water
|
1.0 M NaOH
|
Addn of 0.5 mL HCl
|
Benzoic Acid

|
|
soluble
|
insoluble
|
Problems
1. Explain the results for the tube in which 1.0 M NaOH was added to benzoic acid. Write an equation for this. (Your answer should include both an equation and an explanation.)
2. Now explain what happened when 6.0 M HCl was added to this same tube and provide an explanation for this. Write an equation for this. (Your answer should include both an equation and an explanation.)